Which of the Following Represents a Precipitation Reaction
The balanced net ionic equation is. 2KNO3 s 2KNO2 s O2 g d.

How To Solve Gravimetric Analysis Problem Analysis Solving Solubility
BaC 2 H 3 O 2 2.

. Therefore the correct answer is B. CoCl2 aq Naso aq Coso aq2NaCl aq B. Na 2 SO 4 pbNO 3 to PbSO 4 2NaNO E.
Which of the following represents a precipitation reaction. H 2 SO 4 NaCl to NaHSO 4 HCl B. A The reaction involves a transfer of electrons between reactants.
D Na 2 SO 4 pbNO 3 to PbSO 4 2NaNO. - Which of the following represents a precipitation reaction. Precipitation Reaction CaNO 3 2aq Na 2CO 3aq CaCO 3s 2NaNO 3aq CaNO 3 2and Na 2CO 3 represent ionic compounds.
A H 2 SO 4 NaCl to NaHSO 4 HCl. Ag aq I- aq AgI s A precipitation reaction takes place when aqueous cobalt III chloride reacts with aqueous lithium hydroxide. 2H2 g O2 g 2H2O l b.
When Ag reacts with Cl we get a white precipitate of AgCl that is silver chloride. A 2C 2 H 6 g 7O 2 g 4CO 2 g 6H 2 Ol. A As Bs Cs Ds b As Baq Caq Dl c Aaq Baq Cs Daq d Aaq Bs Caq Dl types of chemical reactions.
CA 2KBr aq Cl2 g 2Cl aq Br2 B2KNO3 s 2KNO2 s O2 g C2H2 g O2 g 2H2O 1 D. A precipitation reaction is possible when two or more aqueous solutions of strong or weak electrolytes are mixed. Which of the following reactions represents a precipitation reaction.
I Displacement reaction ii Precipitation reaction iii Combination reaction iv Double displacement reaction a i only b ii only c iv only d ii and iv - Get the answer to this question and access a vast question bank that is tailored for students. B This type of reaction usually involves ionic compounds. 6H2O l 6CO2 g C6H1206 1.
Which of the following represents a precipitation reaction. Aaq Baq ----- Cs Daq - d. Tamil Nadu Board of Secondary Education SSLC English Medium Class 10th.
Question Bank Solutions 6910. As Bs ----- Cs Ds - b. Which of the following represents a hydrogen displacement reaction.
Which of the following represents a precipitation reaction. AgNO aq KCI aq AgCl s KNO3aq C. CoCl2aq Na soaq Coso aq2NaClaq B.
A precipitation reaction is basically another term for a double replacement reaction. A precipitation reaction is a type of chemical reaction that forms a gaseous product such as CO2. Only ionic compounds will react to form precipitates in a.
Water is formed along with Calcium acetate which is a white colored precipitate. CaCl2 aq H2SO4 aq - CaSO4. Which of the following represents a precipitation reaction.
Which of the following reactions is a precipitation reaction. A 2H2g O2g 2H2Ol B CaBr2aq H2SO4aq CaSO4s 2HBrg C 2KNO3s 2KNO2s O2g D 2KBraq Cl2g 2KClaq Br2l E 2Als 3H2SO4aq Al2SO43aq 3H2g. Which of the following reactions represents a precipitation reaction.
A HNO2 aq NaOH aq NaNO2 aq H2O 1 B 2CHOH U 702 g 4CO2 g 6H2O 1 C 2H2O2 1 2H2O l O2 g D FeSO4 aq K S aq FeS s. Which of the following represents a precipitation reaction. 6H2O l 6CO2 g C6H1206 1 6O2 g2C2H6 g 702 g 6H2O g CO2 gO HCl aq KOH aq H2O l KCl aqCaCl2 aq Na2CO3 aq CaCO3 s 2NaCl aq Which of the following reactions represents a precipitation reaction.
As Baq ----- Caq Dl - c. Categorize the following reaction as an acid-base neutralization precipitation combination decomposition combustion displacement or disproportionation reaction. 2Al s 3H2SO4 aq Al2 SO43 aq 3H2 g Expert Answer.
Fe H 2 SO 4 to FeSO 4 H 2. When an insoluble compound it forms a precipitate. H2SO4 NaCl to NaHSO4 HCl B.
CuO 2HCl to Cl 2. HNO3 aq NaF aq HF aq NaNO3 aq D. Fe H2SO4 to FeSO4 H2 C.
Which of the following represents a precipitation reaction. Therefore CaBr2aq H2SO4aq - CaSO4s 2 HBrg is a precipitate reaction. Both are precipitation reaction.
HC 2 H 3 O 2 lOH aqH 2 OOC 2 H 3 O 2 aq Option A and D are correct answers. Concept Notes Videos 411. CaBr2 aq H2SO4 aq CaSO4 s 2HBr g A 2000 mL sample of 01015 M nitric acid is introduced into a flask and water is added until the volume of the solution reaches 250.
See the answer See the answer done loading. 4HCl MnO2 to MnCl2 -. As I described in the previous lesson when ionic compounds dissolve in water the ions are separated and they move throughout the liquid like any other particle in the liquid.
Select all that apply. This can also be seen by the fact that an insoluble compound CaSO4 is formed in this reaction. C 4HCl MnO 2 to MnCl 2 2H 2 O Cl 2.
C This type of reaction forms one or more insoluble products. Which of the following correctly represents the type of the reaction involved. D This type of reaction often occurs in aqueous solution.
Asked Oct 20 2020 in Types of Chemical Reactions by Laashya 513k points Which of the following represents a precipitation reaction. B Fe H 2 SO 4 to FeSO 4 H 2. Which of the following represents a precipitation reaction.
Which of the following options correctly describe a precipitation reaction. 1 question Which of the following reactions represents a precipitation reaction. CaBr2 aq H2SO4 aq CaSO4 s 2HBr g c.
AgNO3 aq NaCl s AgCl aq NaNO3 aq 1. E CuO 2HCl to Cl 2 H 2 O. Aaq Bs ---.
Which of the following represents a precipitation reaction. 4HCl MnO 2 to MnCl 2 2H 2 O Cl 2. 2KBr aq Cl2 g 2KCl aq Br2 l e.
AgNO aq KCI aq AgCl s KNO3 aq C. View the full answer. Which of the following concerning precipitation reactions isare correct.
One of the products of this reaction would be.

Which Chemical Equation Represents A Precipitation Reaction A Mg Clo3 2 Aq 2hcl Aq Brainly Com
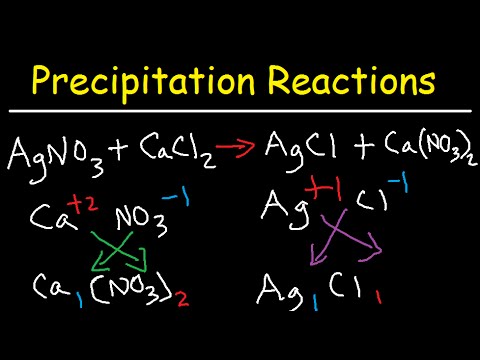
Precipitation Reactions And Net Ionic Equations Chemistry Youtube
Comments
Post a Comment